How Different Types of Macrophages Affect the Progression of Fatty Liver Disease
An accumulation of fat in liver cells is a typical symptom of fatty liver disease (FLD). It includes a range of liver diseases, such as non-alcoholic steatohepatitis (NASH), a more severe type of NAFLD, and non-alcoholic fatty liver disease (NAFLD). Because FLD is linked to obesity, type 2 diabetes, and metabolic syndrome, it has gained international attention as a major public health concern. One aspect of fatty liver disease that has received a lot of attention is the immune system’s participation, specifically with regard to macrophages. Macrophages are immune cells that are important in liver inflammation, tissue remodeling, and fibrosis because of their capacity to absorb and break down pathogens and cellular debris. Gaining knowledge on how various macrophage subtypes affect the progression of fatty liver disease can be extremely helpful in identifying new treatment avenues.
Types of Hepatic Macrophages
The two primary types of macrophages seen in the liver are monocyte-derived macrophages and Kupffer cells. Every type has different etiology, roles in the pathophysiology of fatty liver disease, and functions.
Kupffer Cells: The Macrophages Residing in the Liver
Specialized macrophages called kupffer cells dwell in the liver and make up a sizable component of the immune cell population in the liver. They exist in the liver’s tiny blood veins, or sinusoids, and are arranged in a certain way to interact with blood coming from the stomach. Kupffer cells are able to monitor the liver’s surroundings and preserve homeostasis because of this location.
Kupffer cells carry out vital tasks in a healthy environment, including eliminating pathogens, eliminating apoptotic (dead) cells, and detoxifying dangerous compounds. Additionally, they support immunological tolerance and lessen unneeded inflammation. But in the context of fatty liver disease, oxidative stress, endotoxins originating from the gut, and excess fatty acids can all cause Kupffer cells to become activated. Pro-inflammatory cytokines, including as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6), are produced as a result of this activation. These cytokines exacerbate inflammation, draw in more immune cells, and exacerbate fibrosis and injury to the liver.
Macrophages Derived from Monocytes: The Assigned Support Teams
When the liver is injured or inflamed, it also draws monocyte-derived macrophages from the circulation in addition to Kupffer cells. Chemokines like C-C motif chemokine ligand 2 (CCL2), which are high in fatty liver conditions, attract these monocytes, which are progenitor cells in the circulation, to the liver. Monocytes become macrophages after migrating into the liver tissue, where they support the inflammatory response.
The inflammatory phenotype of monocyte-derived macrophages is frequently higher than that of resident Kupffer cells. They are strong generators of reactive oxygen species (ROS) and pro-inflammatory cytokines, which can worsen inflammation and damage to the liver. The development of NASH, a more severe form of fatty liver disease marked by inflammation, hepatocyte damage, and fibrosis, is linked to the accumulation of these macrophages in the liver. Simple steatosis is the accumulation of fat in the liver.
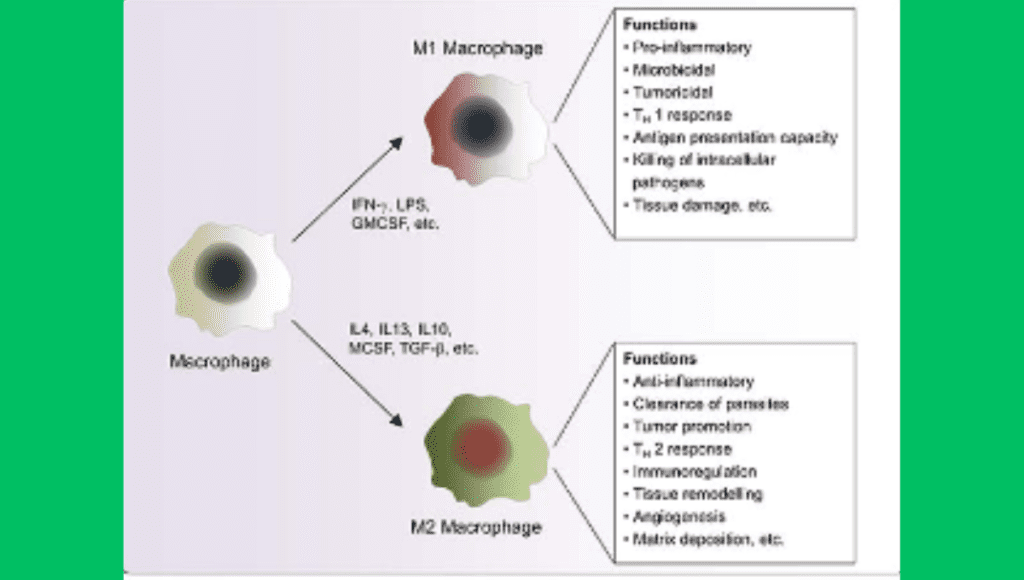
M1 vs. M2 Macrophage Polarization
Macrophages are incredibly malleable cells that can change their functional state in response to a variety of environmental cues. Macrophage polarization, the capacity to alter function, is generally divided into two primary phenotypes: M1 (pro-inflammatory) and M2 (anti-inflammatory). The development and course of fatty liver disease are significantly influenced by the balance between M1 and M2 macrophages in the liver.
Pro-inflammatory phenotype M1 macrophages
Pro-inflammatory stimuli, such as interferon-gamma (IFN-γ) and lipopolysaccharides (LPS) from gut bacteria, activate M1 macrophages. One of the characteristics of these macrophages is that they produce pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α. M1 macrophages aid in the removal of pathogens and diseased cells during the early stages of the immune response to infections and tissue damage.
M1 macrophages contribute to a chronic inflammatory state in fatty liver disease. These macrophages continuously release pro-inflammatory cytokines, which trigger the activation of other immune cells and continue the cycle of inflammation and hepatic injury. Prolonged inflammation can cause damage to the hepatocyte, cause cell death, and activate the stellate cells in the liver, which produce collagen and cause fibrosis. As a result, the development of liver fibrosis and the progression of NAFLD to NASH are tightly associated with M1 macrophages.
M2 Macrophages: A Type of Anti-Inflammatory Macrophage
M2 macrophages are linked to anti-inflammatory and tissue healing processes, in contrast to M1 macrophages. M2 macrophages are distinguished by their generation of anti-inflammatory cytokines, such as interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β), and are activated by signals such as interleukin-4 (IL-4) and interleukin-13 (IL-13). These cytokines aid in tissue remodeling, the reduction of inflammation, and the restoration of injured tissues.
M2 macrophages defend against fatty liver disease by reducing inflammation and aiding in liver rehabilitation. However, by encouraging the hepatic stellate cells to produce extracellular matrix components, overactivation of M2 macrophages can also lead to liver fibrosis. Consequently, even though M2 macrophages are usually thought to be helpful in reducing inflammation, their involvement in fibrosis implies that maintaining liver function and preventing liver scarring may require a careful balance.
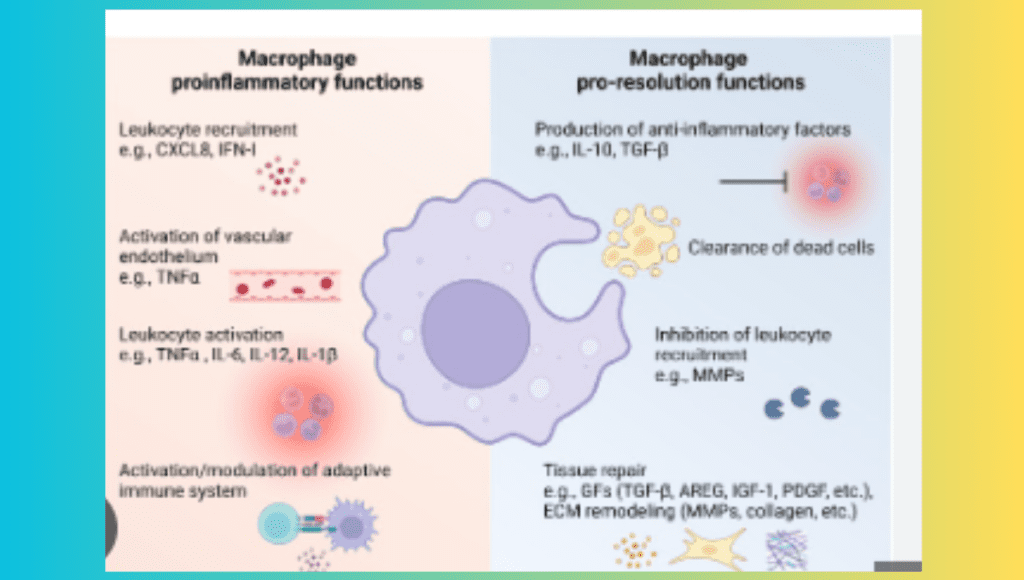
Mechanisms by Which Macrophages Affect Fatty Liver Disease and Inflammation Promotion
The stimulation of inflammation is one of the main ways that macrophages affect the course of fatty liver disease. Endotoxins, ROS, and excess fatty acids are examples of metabolic stresses that can activate monocyte-derived macrophages as well as Kupffer cells. Once activated, these macrophages release a variety of chemokines and pro-inflammatory cytokines that draw lymphocytes and neutrophils, among other immune cells, to the liver. Liver damage, hepatocyte apoptosis (cell death), and the transition from basic steatosis to NASH are all largely caused by this inflammatory response.
Resistance to Insulin
Macrophages emit pro-inflammatory cytokines such TNF-α and IL-6, which lead to the development of insulin resistance, a typical hallmark of fatty liver disease. Reduced insulin sensitivity is the result of these cytokines interfering with hepatocyte, adipocyte, and muscle cell insulin signaling pathways. Insulin resistance increases the rate at which fat is broken down in adipose tissue, a process that raises blood levels of free fatty acids. The liver absorbs these fatty acids and stores them as triglycerides, which causes steatosis. Thus, the buildup of fat in the liver is intimately related to insulin resistance generated by macrophages.
Effects on Metabolism of Lipids
Additionally, macrophages have an impact on the liver’s lipid metabolism, which is crucial to the onset of fatty liver disease. Genes involved in lipid uptake, synthesis, storage, and export can have their expression changed by the inflammatory cytokines that macrophages release. TNF-α, for instance, has been demonstrated to downregulate the expression of genes involved in fatty acid oxidation and lipid export while upregulating the expression of lipogenic enzymes, which stimulate triglyceride synthesis. Hepatocytes accumulate fat as a result of this imbalance in lipid metabolism, which exacerbates liver steatosis.
Development of Fibrosis
An excessive build-up of extracellular matrix proteins is a common result of long-term liver inflammation, which is known as liver fibrosis. Because they activate hepatic stellate cells, the main cell type in the liver that produces collagen, macrophages are essential in the development of fibrosis. Fibrotic scar tissue is created when activated hepatic stellate cells deposit collagen and other matrix proteins. Although, through separate ways, M1 and M2 macrophages both contribute to fibrosis. By producing pro-inflammatory cytokines and reactive oxygen species (ROS) that lead to hepatocyte damage and death and activate stellate cells, M1 macrophages facilitate fibrosis. Conversely, M2 macrophages induce fibrosis by secreting TGF-β, a strong fibrogenic cytokine that directly increases the production of collagen by stellate cells.
Therapeutic Consequences
Targeting macrophage activity and polarization offers a viable treatment approach because of the important role that macrophages play in the onset and progression of fatty liver disease. Numerous possible strategies are being investigated:
Anti-Inflammatory Medication
Treatments that focus on the macrophages’ pro-inflammatory roles may be able to lessen liver inflammation and stop the disease’s progression. For instance, medications that block the synthesis or function of pro-inflammatory cytokines such as TNF-α (such as anti-TNF antibodies) or chemokines that facilitate the recruitment of monocytes (such as CCR2 inhibitors) are being studied for their capacity to lessen fibrosis and inflammation in the liver.
Adjusting the Polarization of Macrophages
Facilitating a transition from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype may aid in the resolution of hepatic inflammation and facilitate tissue regeneration. Using cytokines or other small compounds that stimulate M2 polarization pathways is one way to accomplish this. The risk of encouraging fibrosis must be balanced with the anti-inflammatory effects, though.
Control of Fat Metabolism
Treatments that enhance the liver’s ability to handle lipids can potentially lessen inflammation and macrophage activation. Hepatic steatosis and inflammation can be decreased by medications that, for instance, target important regulators of lipid synthesis, such as sterol regulatory element-binding protein (SREBP), or that promote fatty acid oxidation, such as peroxisome proliferator-activated receptor (PPAR) agonists.
Liver-Gum Axis Adjustment
Potential therapeutic methods include modifying the gut microbiota or improving the integrity of the gut barrier, as gut-derived endotoxins have the ability to activate liver macrophages and increase inflammation. Reducing endotoxin levels and liver macrophage activation may be achieved through the use of probiotics, prebiotics, and dietary modifications that modify the composition of the gut microbiome.
In summary
Macrophages are involved in the development and progression of fatty liver disease through their mediating role in inflammation, lipid metabolism dysregulation, insulin resistance, and fibrosis. The ratio of pro-to anti-inflammatory M2 and pro-inflammatory M1 macrophages affects the liver’s reaction to damage and metabolic stress. Gaining knowledge of the intricate interactions between various macrophage subtypes and their roles can help develop novel treatment strategies for the treatment of fatty liver disease. It appears possible to design effective medicines that can stop the progression of fatty liver disease and enhance patient outcomes by focusing on macrophage activation, recruitment, and polarization.


4 thoughts on “Macrophages and Fatty Liver Disease: Unraveling the Inflammatory Connection”